We streamline the path discoveries take into the clinic with efficient development practices, while focusing our education on improving quality, safety, and seamless teamwork.
Facts & Stats
Houston Methodist
- 8 hospitals
- 2,273,592 outpatient visits
- 148,733 admissions
- 33,663 employees
- 5,115 affiliated physicians
- 842 faculty
- $299 million research & education investment
- 2,570 credentialed researchers
- 41,100 learners
Research Funding
- $202 million in annual research expenditures
- $71 million in annual education expenditures
- $90 million in total extramural funding
- $41 million in National Institutes of Health funding
- $31 million Translational Research Initiative product development fund
Academic Affiliations & People
- 842 faculty
- 2,570 credentialed researchers
- Affiliated with Weill Cornell Medical School
Research
- 232 licensable technologies
- 22 products in the pipeline
- 1,900 clinical protocols
- 760 active clinical trials
- 212 investigator initiated trials
- 2,000 peer-reviewed publications
- 136 collaborating countries
- 6,690 global collaborations
- 19 Interdisciplinary Departments: Anesthesiology & Critical Care; Cardiology; Cardiovascular Sciences; Cardiovascular Surgery; Medicine; Nanomedicine; Stanley H. Appel Department of Neurology; Neurosurgery; Obstetrics & Gynecology; Ophthalmology; Oral & Maxillofacial Surgery; Orthopedic Surgery; Otolaryngoloy; Pathology & Genomic Medicine; Psychiatry and Behavioral Health; Radiation Oncology; Radiology; Surgery; Urology
- 19 Interdisciplinary Centers: Cellular Therapeutics; Bioenergetics; Cardiovascular Regeneration; Health Data Science and Analytics; Human Performance; Critical Care; Health and Nature; Robotics, Imaging and Navigation; Immunobiology and Transplant Science; Infectious Diseases; Brain and Pituitary Treatment; Musculoskeletal Regeneration; Alzheimer’s Disease; Neuroregeneration; Neural Systems Restoration; Rapid Device Translation; RNA Therapeutics; Liver Disease and Transplantation
Education
- EnMed: Texas A&M University School of Engineering Medicine at Houston Methodist combined MD and master's in engineering program
- Master's in Clinical Translational Management with University of St. Thomas
- 71 GME programs; 388 residents & fellows
- 41,100 total learners
- 1,230 trainees in residence for medicine, research, nursing, pharmacy, and allied health
- Houston Methodist Institute for Technology, Innovation & Education (MITIE℠)
Consortia & Collaborative Centers
-
Gulf Coast Consortium
-
Center for Translational Neural Systems Restoration, in partnership with Rice University
-
Center for Human Performance, in collaboration with Rice University
-
Center for Cell and Gene Therapy, in partnership with Baylor College of Medicine and Texas Children’s Hospital
-
Center for Health and Nature, in partnership with Texas A&M University and Texan by Nature
-
Center for Rapid Device Translation, in partnership with J&J Innovation Labs
-
Siemens Imaging Consortium, based at Houston Methodist with six local universities and medical centers
Technology
- Ann Kimball & John W. Johnson Center for Cellular Therapeutics to translate laboratory discoveries into cutting-edge cellular therapies for patient care
- Center for RNA Therapeutics provides access to state-of-the-art for RNA manufacturing and technology
- Imaging innovation hub: A Siemens and Houston Methodist led consortium with Rice University, Texas A&M, UT Health Science Center at Houston, UT Medical Branch at Galveston, University of Houston, and Baylor College of Medicine
- 7 Tesla MRI and MAGNETOM Terra, allowing for unprecedented visualization of anatomical details, physiology and biological function
- Cyclotron and 9 ventilated hot cells (chemistry labs in lead boxes) to produce clinical grade and rare custom radiopharmaceuticals for advanced diagnostic and therapeutic imaging
- Inveon dedicated PET system, Inveon multimodality SPECT/CT system, Caliper IVIS-200 system, and Maestro in vivo fluorescence imaging system
- Good Laboratory Practice (GLP) facilities perform risk, safety and efficacy assessment studies in compliance with FDA guidelines in preclinical models
- Current Good Manufacturing Practices (cGMP) facilities to produce pharmaceuticals, vaccines and nanoparticles for testing and research
- Cockrell Center for Advanced Therapeutics Clinical Research Phase I Unit to support clinical investigators and sponsors with early phase and proof-of-concept clinical trials
- 21 core facilities for access to pioneering technology and data analysis, including: advanced cellular and tissue microscopy; biorepository; biostatistics and bioinformatics; clinical research services; comparative medicine; digital solutions; electron microscopy; event services; flow cytometry; high performance computing; immunomonitoring; intravital microscopy; machine shop; magnetic stimulation devices; nanoengineering; preclinical catheterization lab; research pathology; RNAcore; translational imaging; translational production & quality
Our Research Philosophy
Because most innovation occurs at the intersection of disciplines rather than within a silo, we have structured the research enterprise in a way that allows us to create interdisciplinary points of connection by providing platform technology support to clinical research programs throughout Houston Methodist. This intentional blending of departmental boundaries adds a new level of innovative, collaborative research, conducted by teams of scientists from a variety of disciplines and backgrounds.
The various medical disciplines intersect at one or all of three broad strategic areas — precision medicine, biotherapeutics and restorative medicine and outcomes, quality and health care performance.
 Precision medicine
Precision medicine
 Biotherapeutics and Restorative Medicine
Biotherapeutics and Restorative Medicine
 Outcomes, Quality and Health Care Performance
Outcomes, Quality and Health Care Performance
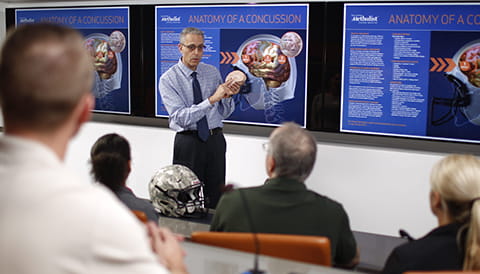
Outcomes, quality and health care performance uses interdisciplinary research to improve outcomes for patients in acute care settings. By improving clinical practice, supporting patient decision-making, evaluating innovative therapeutic procedures and promoting education for health professionals, we ensure that our research discoveries provide the most efficient and effective regimens and practice in clinical settings. Houston Methodist is particularly well positioned to tackle the clinical challenge of delivering high-quality, cost-effective care, as an organization with a broad network of private and employed physicians with notably diverse patient populations.


