Spinal Cord Injury Researchers Develop New Model to Stimulate Upper Limb Function in Rats
Jan. 7, 2022 - Eden McCleskeyIn spinal cord injury research, much like in spinal cord clinical rehabilitation settings, success doesn't happen overnight. It typically only comes after long periods of hard work, making it that much more impactful when it occurs.
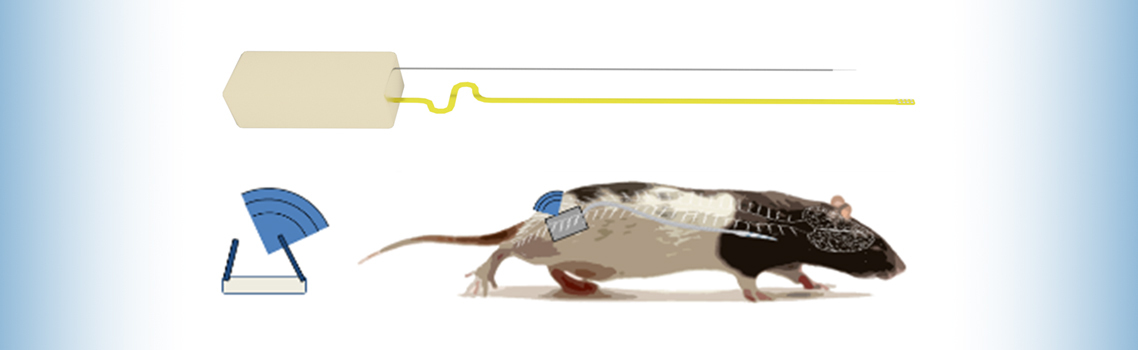
For a team of neurosurgical researchers at Houston Methodist, the most recent breakthrough comes in the form of a wireless spinal stimulation system that overcomes the limitations and side effects of previous models.
Electrical stimulation has gained traction in both clinical and research settings as a way to induce neural plasticity and improve functional recovery following spinal cord trauma, but the exact mechanisms of action and long-term recovery effects remain unclear. More robust research in animal models is paramount, particularly the design of a more effective electrical stimulation system for rats.
The team's novel approach for epidural stimulation of the spinal cord using a fully implantable stimulation system and ventral electrode array was recently described in the Nature journal Scientific Reports. The study was led by Dr. Philip Horner, a Houston Methodist professor of neuroregeneration, and funded by Wings for Life, a spinal cord research foundation.
Solving a critical access issue
One of the team's most important goals was to safely and effectively target the cervical motor region of the spine, which controls arm, hand and finger movements.
"For a patient with a cervical spinal cord injury, focusing on improving hand and arm function often has a far greater impact on quality of life than improving gross motor skills like standing and walking," says Dr. Sean Barber, a Houston Methodist neurosurgeon and study co-author. "It makes a big difference in the independent ability of patients to eat, work, use a computer or cell phone, go to the bathroom and move around in their wheelchair or in their house."
Researchers historically have had great difficulty stimulating spinal motor neurons because they're located on the ventral, or front-facing, side. Previous models, which relied on intraspinal placement of wire electrodes, caused side effects ranging from neural tissue damage to inflammation and gliosis (scarring) of the spinal cord, potentially offsetting any therapeutic benefits.
"Similarly, if you try to provide epidural stimulation to the ventral side from the easier-to-access dorsal side, you have to use a higher voltage, which also causes tissue damage, inflammation and scarring," Dr. Barber explains.
Instead, the Houston Methodist team used electrophysiologic mapping of the ventral spinal cord to target muscle groups of the rat forelimb, testing which stimulation protocols could achieve unique activation patterns.
Eventually, the team pinpointed a single electrode lead position that enabled stimulation of the motor neurons within the cervical spinal cord. They also developed a precise technique for placing electrodes in the ventral epidural space using a dorsal surgical approach. The technique involves performing a hemilaminectomy at the T4/T5 junction, sliding under the T4 root and carefully progressing to the relevant level.
"Our aim was to develop a system with not only the feasibility and safety advantages of a dorsal epidural stimulator, but one that could effectively target ventrally located motor-associated circuitry previously only accessible by intraspinal systems," says Dr. Horner. "The system and implantation technique we demonstrated allowed for experimentation with a unique path associated with motor circuitry of the upper limb and hand while minimizing collateral epidural and parenchymal scarring."
Wireless design for enhanced mobility
Another important goal for the research team was to create a fully wireless implant that would be suitable for long-term stimulation strategies in awake, freely functioning animals.
Rat models of spinal cord injuries typically involve inducing an injury on only one side, keeping the other side as a control.
"Previous iterations in rat models used tethered systems, where the rat has a wire coming out of their neck that connects to an external power source," Dr. Barber says. "This is how you stimulate, or shock, the area, how you control the level of stimulation. But the problem is this severely limits the rat's mobility and what we can learn about how the stimulation affects the paralyzed side versus the control side."
To combat this issue, the team worked with Houston Methodist engineer and study co-author Matt Hogan, Ph.D., to develop a fully implantable 22 mAh lithium ion battery and inductive wireless recharging base that:
- is biocompatible, well tolerated and operates for up to 6 months
- communicates with a central hub for implant status monitoring and protocol adjustment
- unilaterally delivers stimulus to the left or right ventral aspect of the cervical spinal cord
- delivers fully programmable stimulus patterns with control of anodic and cathodic current amplitudes and pulse widths, inter-stimulation intervals (ISI) and independent and programmable selection of anode and cathode among all channels
Similar implants are used in humans for pain relief and research, but the new model marks the first time a scaled-down version for tiny rat bodies has been developed.
Next steps
"The first step of this research was simply developing this technology," Dr. Barber says. "Now that we are beginning to run experiments using it, we have found it to be a very sophisticated, high-fidelity system that can activate many different paths associated with motor circuitry of the upper limb and hand. Since clearing this first hurdle, we have begun to evaluate the role of this type of stimulation in restoring upper limb functionality for people with spinal cord injuries."