On December 11, 2020, the FDA granted Emergency Use Authorization (EUA) approval for the first COVID-19 vaccine, Pfizer’s mRNA vaccine. On week later, the FDA granted EUA to a second mRNA COVID-19 vaccine by Moderna.
You probably have quite a few questions about these new vaccines, especially since both rely on new technology never before used in human vaccines.
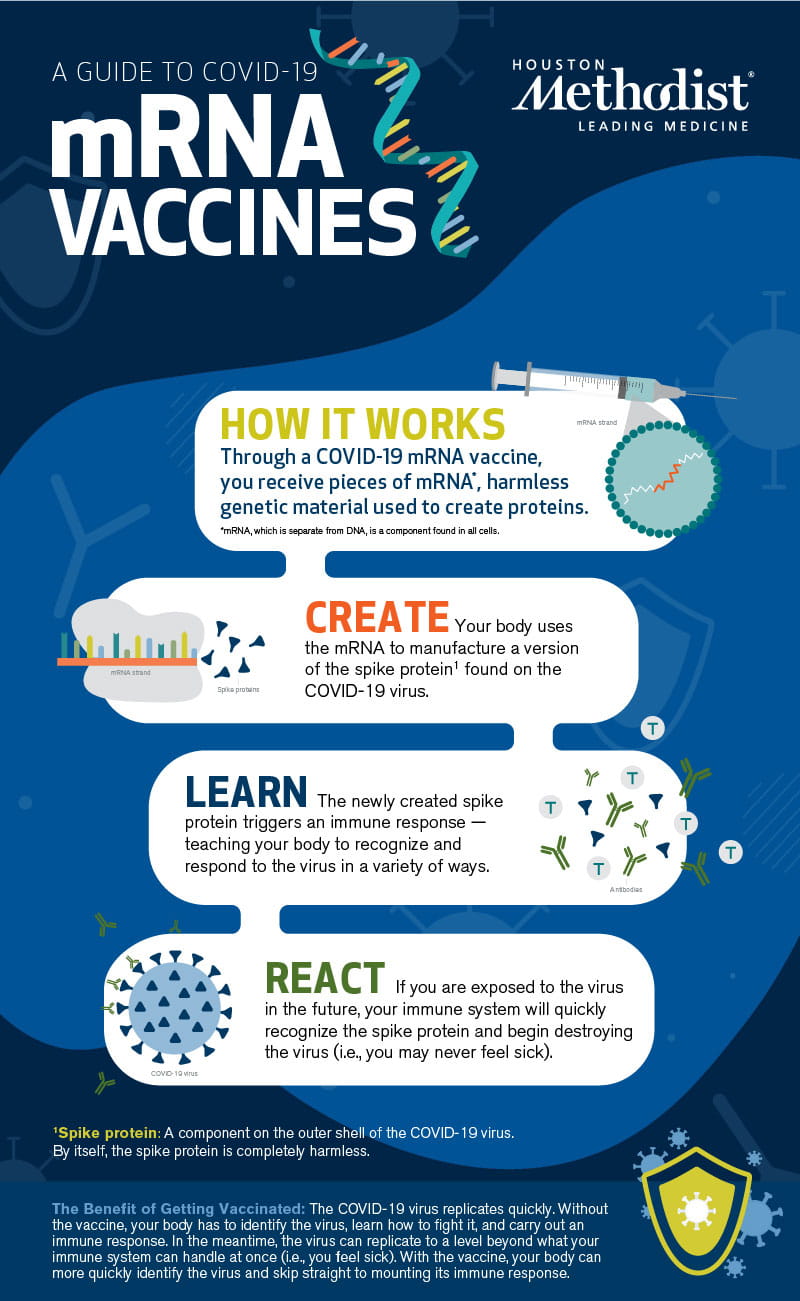
Here's how COVID-19 mRNA vaccines work:
This article was updated on December 19, 2020 to reflect the current state of the COVID-19 vaccine landscape.