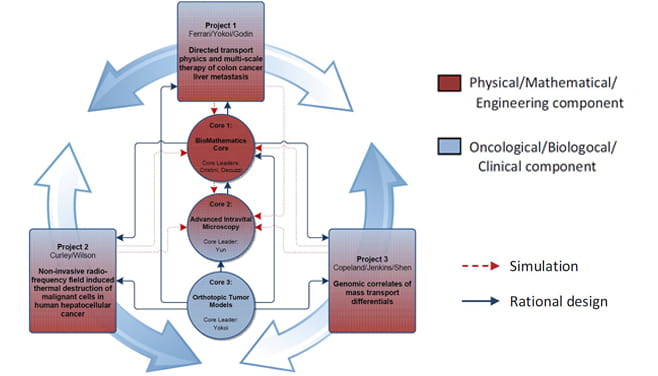
PS-OC Projects & Cores
The Center for Transport Oncophysics (CTO) articulates into three tightly integrated projects under the unifying theme of the quest for a gaining a deeper understanding of the multi-scale differentials in transport properties that accompany — and, in several ways, define — the evolution of malignancies. These differentials manifest themselves with the emergence or the modulation of pathology-associated biophysical and biological barriers to transport. The operational corollary to the unifying basic science theme of this proposed CTO is the quest for innovative, physical science-based methodologies that afford the exploitation of these differentials for improved diagnostics and treatment of cancer.
Physical Sciences — Oncology Center Project 1
Directed transport physics and multi-scale therapy of colon cancer liver metastasis
Project Leaders: Mauro Ferrari, PhD, Biana Godin Vilentchouk, PhD
The overall goal of Project 1 is to develop a broader understanding of the physical barriers and biological factors involved in the progression of liver metastasis in orthotopic tumor models of colorectal cancer (CRC) and to design novel biocompatible delivery carriers able to overcome or take advantage of these barriers with favorable pharmacokinetics and tissue distribution profiles for the highly efficient delivery of novel therapeutic and imaging agents. A physics- and biology-driven and mathematics-based design of engineered drug delivery vectors will multiply the probability of recognition of the novel targets, providing a synergistic solution for imaging and therapy of CRC liver metastasis within the interface of physics, engineering, mathematics and cancer biology.
Project 1: Directed transport physics and multi-scale therapy of colon cancer liver metastasis (Ferrari/Fidler): Targeting of multistage carrier to (left) endothelial vessel walls through specific ligands; (right) to phagocytic cells of the liver (Kupffer cells) that preferentially localize to metastatic loci. One of the therapy methods to be investigated includes thermal RF ablation.
Physical Sciences — Oncology Center Project 2
Project Leaders: Steven Curley, MD and Lon Wilson, PhD
Project 2 will explore issues related to the progression kinetics of a primary hepatic tumor (hepatocellular carcinoma or [HCC]), developing a broader understanding of the biophysical barriers involved in the radio frequency (RF) based thermal therapy and MRI/CT imaging of HCC —employing gold nanoparticles (AuNPs) and fullerene particles (nano-C60). The issues explored will include the transport of the nanoparticles towards the lesion; the sufficient and specific accumulation of the nanoparticles within the tumor cells; and the heat generation upon RF activation and the heat transfer to the surrounding tissue. This goal will be achieved through an integrated process where in vitro testing and in vivo studies are combined with predictive in-silico mathematical models.
Physical Sciences — Oncology Center Project 3
Genomic correlates of mass transport differentials Project Leaders: Neal Copeland, PhD, Nancy Jenkins, PhD
Investigators: Eugene Koay, MD, PhD and Karen Mann, PhD
Project 3 explores the heterogeneity in transport barriers of pancreatic cancer as a direct result of multiple genetic aberrations seen in the disease. Furthermore, the effect of these most relevant genes/pathways on the transport phenotype is dependent on the specific stage of tumor development (i.e., localized, locally advanced and metastatic), with the most pronounced transport differentials anticipated to occur at early stages of tumor development. The transport phenotype offers a mechanistic explanation for the clinical observations associated with prognostic genomic biomarkers and that this physical sciences-driven analysis will help identify novel biomarkers. Moreover, the analysis provides the basis for the rational design of innovative therapeutic strategies. Herein, we aim to characterize the transport phenotype as part of a broader approach for personalized medicine: the biophysical marker.
Cores
Each of the three projects is supported by the following cores:
PS-OC CTO Core 1
BioMathematics Core. Core Leaders: Vittorio Cristini, PhD and Paolo Decuzzi, PhD
Biomathematics Core
This core provides mathematical tools to model and predict the behavior of small molecules and nano-sized particulate systems. It will examine the following:
- Transport dynamics within the authentic patient-specific vasculature accounting for the specific/non-specific adhesive interactions with the vascular endothelium and for the permeability of the vessel walls to both plasma and blood-borne agents
- Extravasation from the vascular compartment to the extravascular matrix through active (transcytosis) and passive (intravascular gaps) mechanism
- Transport across the extravascular matrix and distribution within the tumor microenvironment
- Control of the tumor growth and angiogenic response
- Heat generation through remote RF/NIR activation with modeling of thermal cell damage and apoptosis.
PS-OC CTO Core 2: Advanced Intravital Microscopy Core
Core Leader: Seok H. (Andy) Yun, PhD
The broad goals of the Advanced Intravital Microscopy Core are to provide the Physical Sciences — Oncology Center (PSOC) projects with a number of unique advanced optical systems, to collaborate with the project investigators in the design, execution and analysis of animal experiments, as well as to develop new instrumentation and methodology as needs arise.
The core will be established in Boston within the Wellman Center for Photomedicine at Massachusetts General Hospital (MGH) under Seok-Hyun (Andy) Yun (Harvard University/Mass General Hospital). The laboratory space is well suited for live animal imaging, with its own dedicated murine facility connected to the imaging facility on the same floor, and proximity to the MGH animal facility located in the basement of the building. Routine intravital microscopy experiments will be carried out in Houston within the new Center for Advanced Biomedical Imaging Research (CABIR) building where a room for this activity has been allocated and specifically designed.
Figure 1: Side-view endomicroscopy. Longitudinal cellular imaging can visualize the progression of colorectal tumors and the microenvironment in vivo during premalignant as well as metastatic stages. Images on the right side show the GFP+ human colorectal carcinoma cells (green) implanted in the colon and the vessels (red) in the periphery of the tumor.
PS-OC CTO Core 3: Orthotopic Tumor Models.
With the progress of the Physical Sciences – Oncology Center (PSOC) activities, there is clear need for a centralized core for models that can support projects and cores. This core was initiated in 2011 to provide services for the establishment of orthotopic tumor models.
Core 3 is providing orthotopic models using various types of cancer cells to collaborate with the project investigators in Project 1. The core is also establishing liver metastasis models not only of colon cancer (KM12SM, human colon cancer; CT26, murine colon cancer), but also lung cancer (3LL, murine lung cancer; PC14, human lung cancer), breast cancer (4T1, murine breast cancer), pancreatic cancer (L3.6pl human pancreatic cancer) and melanoma (K1735 and B16, murine melanoma).
Biana Godin-Vilentchouk, PhD (Houston Methodist Research Institute — Houston, Texas)