Masdeu Lab

About Our Lab
Using the tools of human neuroimaging, including magnetic resonance imaging (MRI), positron emission tomography (PET) and electroencephalography (EEG), the neuroimaging lab aims to clarify the neurobiology and improve the treatment of the following disorders causing cognitive impairment.
Alzheimer’s disease
- Timing of beta-amyloid and tau deposition in preclinical stages
- Genetic effects
- Effect of treatment
- Correlation of tau imaging with postmortem tau quantification
Frontotemporal dementia
- Genetic underpinning of regional involvement patterns
- Neuroimaging correlates of social cognition
Traumatic brain injury
- Amyloid and tau imaging in acute and chronic concussion
- Timing and brain localization of tau deposition in patients with repeated head trauma
Schizophrenia
- Anti-synaptic antibodies and their effect on functional connectivity
Working with the MRI and PET facilities in the Translational Imaging Core of the Houston Methodist Research Institute and with the collaboration of a number of external researchers, the lab has utilized and implemented the following techniques:
MRI-related
- Structural MRI
- BOLD MRI
- Activation
- Resting, with functional connectivity
- Diffusion tensor imaging (DTI)
- Susceptibility weighted imaging (SWI)
- Arterial spin labeling (ASL)
PET
PET Radiopharmaceuticals
- [18F] Fludeoxyglucose
- [11C] Pittsburgh compound B
- [18F] T807 (AV-1451)
- [18F] Fluoro-L-DOPA
- [11C] PBR28
11,907
Citations
45
h-index
Open Positions
Houston Methodist in Houston, Texas is a leading academic hospital system dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. Throughout the department of neurology at Houston Methodist, our mission is to deliver superb patient care, to apply state-of-the-art translational research methods to discover the causes of and treatments for human nervous system disorders and to educate each generation of medical students, neurology residents, and postdoctoral fellows. By fostering cross-disciplinary interactions among scientists around the world, we accelerate the pace of discovery and champion global health initiatives. For any positions at Houston Methodist, the first step is to apply online. To find out about open positions, please visit Houston Methodist’s career page and select search openings. The neuroimaging lab often has opportunities available.
Research Assistant:
Primary responsibilities include neuroimaging data analysis, behavioral analysis, data management, and general scientific programming. Standard software tools will be used for the statistical analysis of structural and functional magnetic resonance imaging (MRI), as well as positron emission tomography (PET). A strong background in computer programming and Linux is essential. Applicants must hold a bachelor’s or master’s degree in computer science, engineering, math or physics, although other backgrounds with requisite experience will be considered. Proficiency in Matlab, shell scripting, and python is strongly encouraged. Familiarity with common neuroimaging tools is a plus (Freesurfer, FSL, AFNI, SPM, Slicer, etc.). The candidate is also expected to assist in the management of neuroimaging data, therefore, database experience (MySQL, PostgreSQL) and/or experience with neuroinformatics software (XNAT) is highly beneficial. The ideal candidate must be mature and responsible, with excellent organizational, as well as oral and written communication skills. You must be able to work independently in a fast-paced environment, juggle and prioritize multiple tasks, and seek help when required.
For more information call 713.441.1150 or e-mail alzheimer@houstonmethodist.org
Research Assistant:
Primary responsibilities include neuroimaging data analysis, behavioral analysis, data management, and general scientific programming. Standard software tools will be used for the statistical analysis of structural and functional magnetic resonance imaging (MRI), as well as positron emission tomography (PET). A strong background in computer programming and Linux is essential. Applicants must hold a bachelor’s or master’s degree in computer science, engineering, math or physics, although other backgrounds with requisite experience will be considered. Proficiency in Matlab, shell scripting, and python is strongly encouraged. Familiarity with common neuroimaging tools is a plus (Freesurfer, FSL, AFNI, SPM, Slicer, etc.). The candidate is also expected to assist in the management of neuroimaging data, therefore, database experience (MySQL, PostgreSQL) and/or experience with neuroinformatics software (XNAT) is highly beneficial. The ideal candidate must be mature and responsible, with excellent organizational, as well as oral and written communication skills. You must be able to work independently in a fast-paced environment, juggle and prioritize multiple tasks, and seek help when required.
For more information call 713.441.1150 or e-mail alzheimer@houstonmethodist.org
Research in Methodology
Clinical Research in Methodology
Clinical Research in Methodology
Contact Us
For more information call
713.441.1150 or email
alzheimer@houstonmethodist.org
CONTACT US


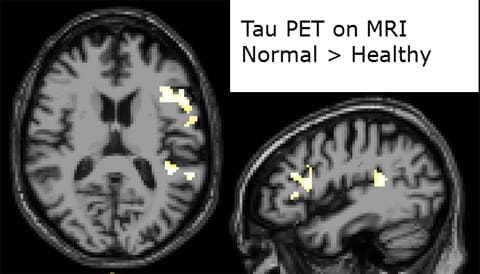
![Fig 4: [18F]AV-1451 PET shows greater uptake in off-target regions in older vs. younger adults](https://www.houstonmethodist.org/academic-institute/-/media/images/research/labs/neurology-masdeulab/fig4.ashx)